This site is intended for Australian Healthcare Professionals.
This site is intended for Australian Healthcare Professionals.



Despite a landscape clouded in complexity,
established and emerging biomarkers are expanding
our view of patient populations, and biomarker
testing could provide a more comprehensive patient
profile and lead to more informed decisions.


This list of gastric cancer biomarkers is not exhaustive. Other biomarkers may exist that are not mentioned on this website.
CLDN18.2=Claudin18.2; FGFR2b=fibroblast growth factor receptor 2b; HER2=human epidermal growth factor receptor 2; MSI=microsatellite instability; PD-L1=programmed death-ligand 1.
AN UNMET NEED
The 5-year survival rate of gastric cancer in Australia is 38%.1

In Australia, approximately 2,500 new cases of stomach cancer are diagnosed each year, with around 1,100 deaths.1
EMERGING BIOMARKERS
help identify previously undefined subsets of mG/GOJ cancer patients:
ESTABLISHED BIOMARKERS
are used to inform clinical decisions:
Most emerging and established biomarkers can be detected by using standard, widely accepted assays such as IHC
EMERGING BIOMARKERS
CLDN18.2:
IHC11*
FGFR2b:
IHC, NGS12,13*†
ESTABLISHED BIOMARKERS
PD-L1:
IHC14–17*‡
HER2:
IHC, ISH, NGS14–17*§
MSI/MMRd:
PCR, NGS/IHC14–16*
IHC=immunohistochemistry; ctDNA=circulating tumour DNA; ISH=in situ hybridisation; MMRd=mismatch repair deficient; NGS=next generation sequencing; PCR=polymerase chain reaction.
* Expression utilises IHC and PCR tests;11,14–16 amplification utilises ctDNA, ISH and NGS tests.13–17
† IHC detects FGFR2b overexpression; NGS detects FGFR2 gene amplification by ctDNA.13,14
‡ Varying diagnostic assays.18
§ Other ISH methods (FISH=fluorescent ISH; SISH=silver ISH; CISH=chromogenic ISH; DDISH=dual-colour dual-hapten ISH).17
Emerging biomarkers are highly prevalent among mG/GOJ biomarkers.
Biomarker prevalence estimates from select studies are reported below. Prevalence data can vary amongst studies due to tumour heterogeneity, differences in patient population, clinical trial methodology, and diagnostic assays used.8,19–21
EMERGING BIOMARKERS
CLDN18.211,19,22
(high expression)‖¶
33–38%
FGFR2b20
(positive)
30%
‖ 2+/3+ staining by IHC in ≥70% of tumour cells. Includes locally advanced unresectable or mG/GOJ adenocarcinoma.11
¶ High expression: 2+/3+ IHC staining in ≥75% of tumour cells.19
ESTABLISHED BIOMARKERS GLOBAL PREVALENCE RATES
PD-L121,23
(variable due to multiple factors)**
CPS ≥1: 67-73%
CPS ≥5: 29-31%
CPS ≥10: 16-18%
HER2
(Positive)
22%8
MSI
(MSI-high)
4-6%24
CPS=combined positive score; mG/GOJ=metastatic gastric/gastro-oesophageal junction.
**PD-L1 prevalence at various CPS thresholds is still being explored. Data are from a randomised controlled trial and a real-world, retrospective medical records study.21,23
Timeline of targetable biomarker discoveries25–29


In 2021, ASCO recognised molecular profiling in gastrointestinal cancer as Advance of the Year.30
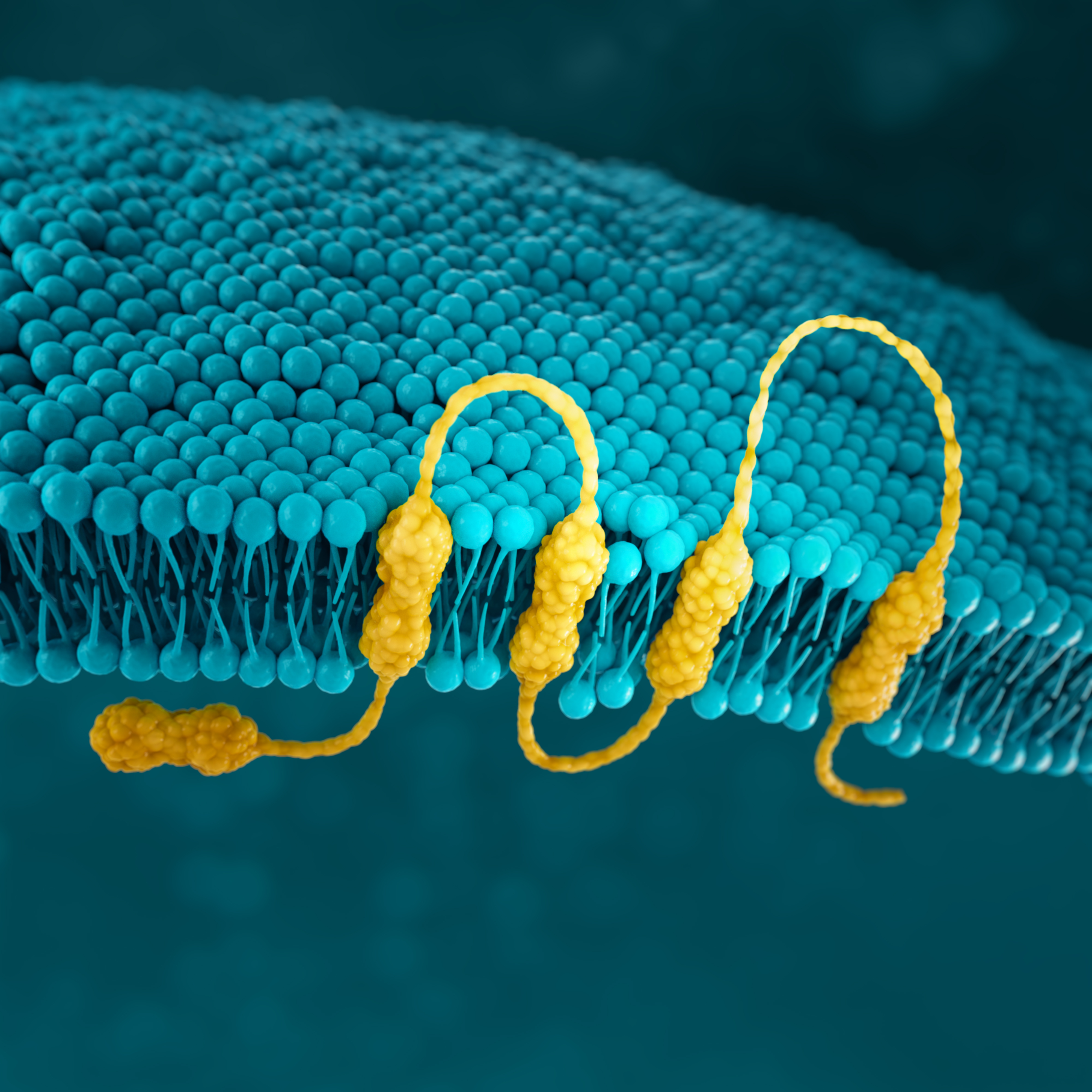
CLDN18.2
Claudins are a family of transmembrane proteins:2,28
Claudins are a major component of epithelial and endothelial tight junctions, which are involved in controlling the flow of molecules between cells.2–4
Claudins are present throughout the body, but two specific splicing isoforms of CLDN18 are localised to certain tissue types:2,28
Preclinical studies have shown that CLDN18.2 may become more exposed and accessible to antibodies as gastric tumors develop.2,32,33
CONFINED IN HEALTHY TISSUE
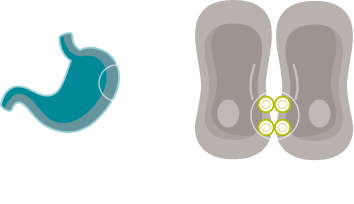
In gastric epithelial cells, CLDN18.2 is typically buried in the tight junction supramolecular complex.2,4,33
It functions to regulate selective barrier properties and contributes to cell-to-cell epithelial adhesion.2–5
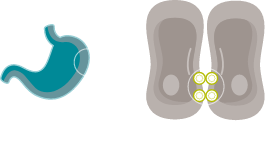
EXPOSED IN TUMOURIGENESIS


Malignant transformation leads to polarity disruptions and structure loss.32,33 As a result, CLDN18.2 may be more exposed and accessible to antibodies.2,32,33
RETAINED DURING TRANSFORMATION


The presence of CLDN18.2 is retained throughout malignant transformation, both in the primary tumour site and metastatic disease.2,32
Although not present in healthy tissues beyond gastric epithelial cells, CLDN18.2 may become activated in oesophageal, pancreatic, lung, and ovarian tumours as well.2
While approximately 70% of locally advanced and mG/GOJ cancers express CLDN18.2 (at any level), recent studies have shown approximately 38% of mG/GOJ patients are CLDN18.2 positive (high expression).19,22
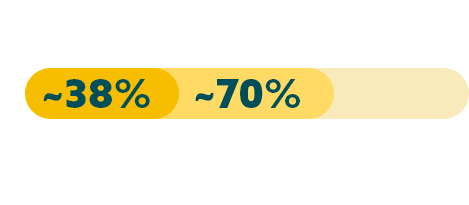

Few patients with locally advanced or mG/GOJ cancer who are CLDN18.2+ (high expression*) also test positive for other biomarkers.19
When evaluating the relationship between CLDN18.2 and other biomarkers, current data suggest there is limited overlap.








* High expression levels of CLDN18.2: 2+ and 3+ intensity in ≥75% tumour cells.19
† Study population was limited to 350 Caucasian patients with mG/GOJ cancer, of which 117 patients had high expression of CLDN18.2. FGFR2b was not evaluated in this study.
CLDN18.2 is expressed in both diffuse-type tumours and intestinal-type tumours.4
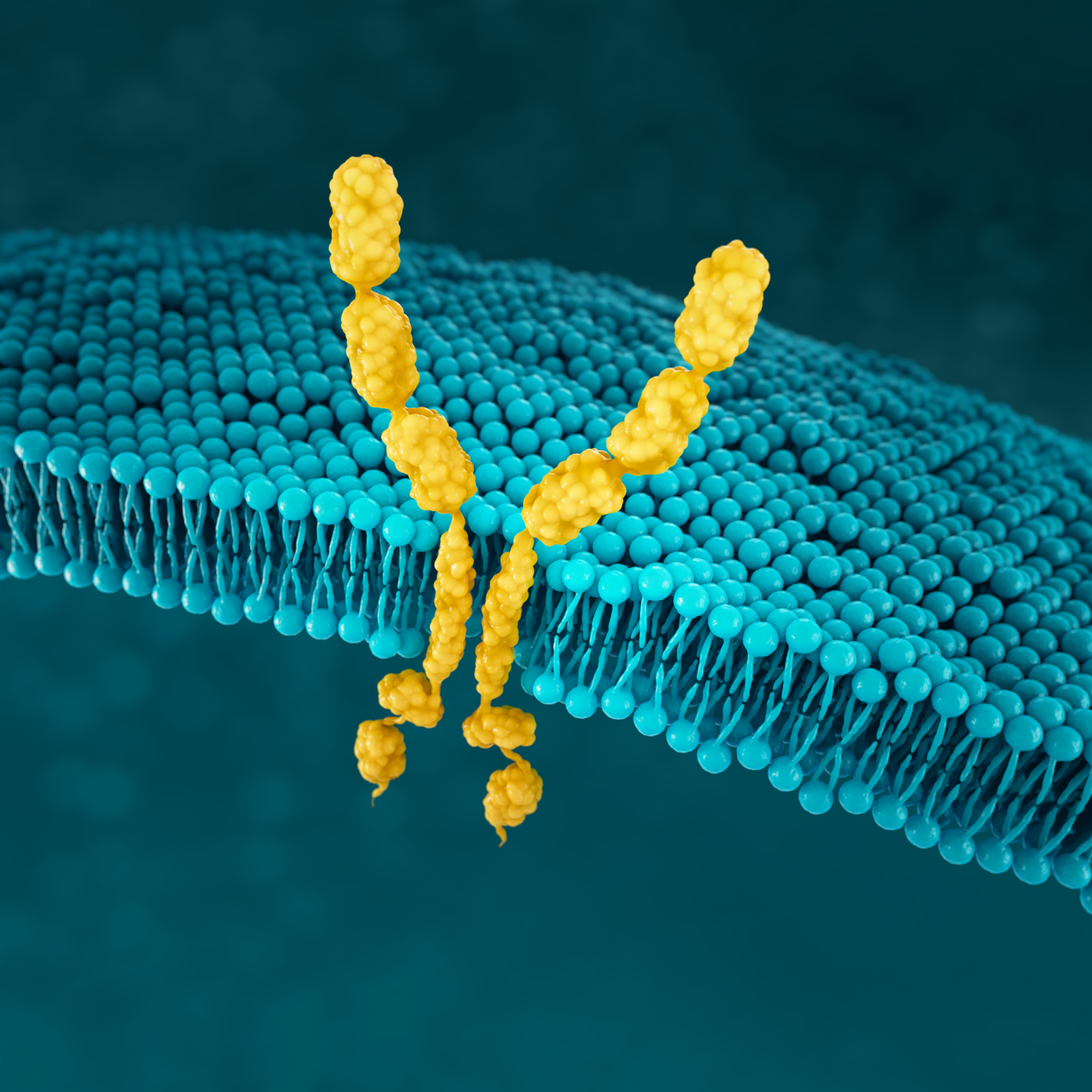
FGFR2b
FGFR2b is a receptor tyrosine kinase that has a role in normal cell development36
FGFR2 amplification is significantly associated with FGFR2 receptor overexpression in gastric cancer39
NORMAL CELL
In normal cells, FGFR signalling is an essential component for cell development. Deregulated FGFR2 pathway, through mutations or translocations, plays a critical role in several tumour types.40
GASTRIC CANCER CELL
FGFR2 overexpression and up-regulated signalling may be key events in a subtype of gastric cancer. Detection of FGFR2 amplification has been the mainstay for pre-screening patients for FGFR2 receptor overexpression.40
Detection of specific FGFR2 isoforms (e.g. FGFR2b) or FGF ligand overexpression may represent novel and enriching predictive biomarkers for gastric cancer40
FGFR2b positivity can be observed in 30% of mG/GOJ cancers.20


FGFR2b positivity: overexpression (IHC) and/or gene amplification by ctDNA (NGS).
Detecting FGFR2b can be done with the following tests:13,14
FGFR2b is a relatively new biomarker in gastric cancer and, therefore, sparse data are available regarding its overlap in expression with other biomarkers
| Biomarker | Biomarker | Prevalence overlap | Reference |
|---|---|---|---|
| FGFR2b | CLDN18.2 | Unknown* | Presently unavailable* |
| FGFR2b | PD-L1+ | Unknown† | Presently unavailable† |
| FGFR2 | HER2 (3+) | 0.3% | Su 201441 |
| FGFR2b | MSI/MMR | Unknown‡ | Presently unavailable‡ |
* A PubMed search using the search terms (gastric cancer) AND (FGFR2) AND (CLDN18.2) produced no articles.
† A PubMed search using the search terms (gastric cancer) AND (FGFR2) AND (PD-L1) produced five articles, none of which included information pertaining to the prevalence overlap of these two biomarkers.
‡ A PubMed search using the search terms (gastric cancer) AND (FGFR2) AND (MSI) produced seven articles, none of which included information pertaining to the prevalence overlap of these two biomarkers. A search using the search terms (gastric cancer) AND (FGFR2) AND (MMR) produced two articles, neither of which included information pertaining to the prevalence overlap of these two biomarkers.
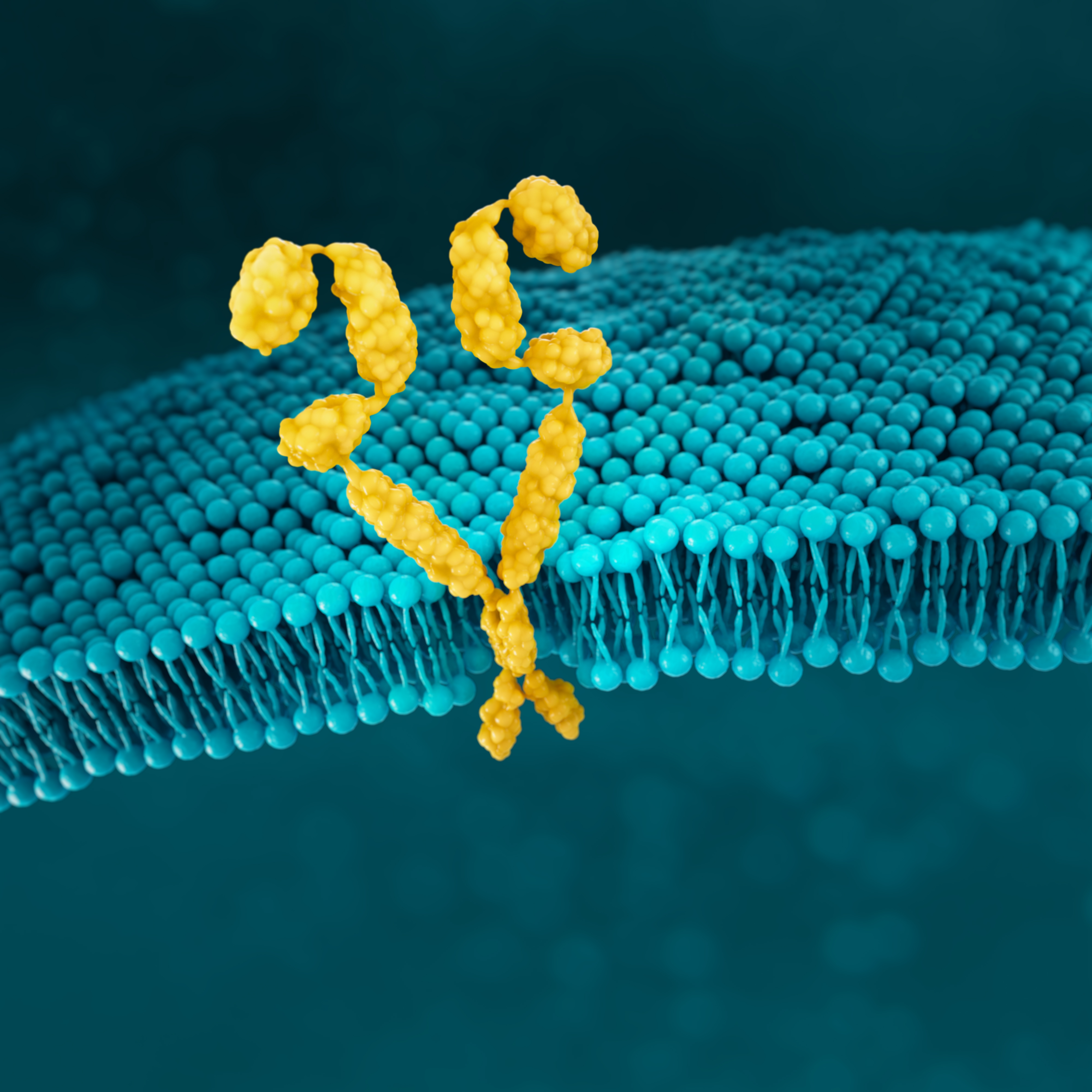
HER2
HER2 is a receptor-tyrosine kinase that is overexpressed and/or amplified in mG/GOJ cancer.6
HER2 is a proto-oncogene that is involved in signalling pathways, which leads to cell growth and differentiation.17
In normal cells, few HER2 receptors exist at the cell surface (see figure below) so few heterodimers are formed, and growth signals are relatively weak and controllable43
NORMAL CELL
Normal cell with low HER2 protein receptor expression and few HER2 receptor heterodimers at the cell surface43
HER2+ CANCER CELL
HER2 is overexpressed and/or amplified, multiple HER2 receptor heterodimers are formed at the cell surface and cell signalling gets enhanced.43
When HER2 is overexpressed, multiple HER2 protein receptors are formed and cell signalling is promoted, which results in enhanced responsiveness to growth factors and malignant growth.43
HER2 positivity has been reported in 22% of advanced G/GOJ cancers.8


Detection of HER2 may be done with IHC, ISH methods and NGS, and is generally more associated with intestinal type tumours.8,14,17*
HER2 positivity: overexpression (IHC3+) and/or gene amplification (FISH-positive).
There is limited overlap between HER2 and other gastric cancer biomarkers with three studies reporting minimal overlap between HER2 and CLDN18.2
| Biomarker | Biomarker | Prevalence overlap | Reference |
|---|---|---|---|
| HER2 | CLDN18.2 | 12-15% | Pellino 2021,19 Moran 2018,44 Schuler 201745 |
| HER2 | PD-L1+ | 7.4% | Angell 201846 |
| HER2 | MSI | 11.1% | Angell 201846 |
| HER2 | FGFR2 | 0.3% | Su 201441 |
CISH=chromogenic ISH; DDISH=dual-colour dual-hapten ISH; FISH=fluorescent ISH; IHC=immunohistochemistry; ISH=in situ hybridization; NGS=next generation sequencing; SISH=silver ISH.
*IHC/ISH should be considered first, followed by additional NGS testing as appropriate.14

MSI
MSI expression is associated with genomic instability and increased susceptibility to tumour development.6
Microsatellites are repeated sequences of nucleotides in DNA.9
The main mechanisms by which failure occurs in the MMR system are via genetic and epigenetic changes in h-MLH1 and h-MSH2, and less frequently in h-MSH6 and h-PMS248
MICROSATELLITE STABLE TUMOUR
TUMOUR WITH HIGH MSI
MMRd=MMR deficiency.
Because somatic mutations in MSI gastric cancers are common, it is difficult to pinpoint the target genes in which mutations lead to MSI gastric carcinogenesis, however, MSI tumours are more prone to exhibit mutations in the oncogenes EGFR, KRAS, PIK3CA and MLK348
In genomically stable tumours, with a functional MMR system, DNA replication errors occur rarely. Conversely, in the presence of high MSI/MMRd, DNA replication errors go undetected and unrepaired, leading to a tumour with a high mutational burden. Such hyper-mutated cancer cells excessively produce mutation-associated neoantigens, which are presented by MHC molecules on the cell surface to stimulate T-cell activation and tumour infiltration by immune cells. To counteract this vigorous immune response, tumour cells expose checkpoint molecules, e.g., PD-L1, to inhibit anti-tumour activity.49,50
PD-L1=programmed death-ligand 1.
MSI-H has been reported in 4% of mG/GOJ cancers.24


MSI-H=MSI-high.
Detection of MSI is typically assessed with various methods.14
MMRd=MMR deficiency.
There is variable overlap of MSI/MMR and other gastric cancer biomarkers; overlap of deficient MMR (dMMR) and CLDN18.2 is minimal
| Biomarker | Biomarker | Prevalence overlap | Reference |
|---|---|---|---|
| dMMR | CLDN18.2 | 15% | Pellino 202119 |
| MSI | PD-L1+ | 53.2% | Angell 201846 |
| MSI | HER2 (3+) | 4.2% | Angell 201846 |
| MSI/MMR | FGFR2b | Unknown* | Presently unavailable* |
*A PubMed search using the search terms (gastric cancer) AND (FGFR2) AND (MSI) produced seven articles, none of which included information pertaining to the prevalence overlap of these two biomarkers. A search using the search terms (gastric cancer) AND (FGFR2) AND (MMRd) produced two articles, neither of which included information pertaining to the prevalence overlap of these two biomarkers.
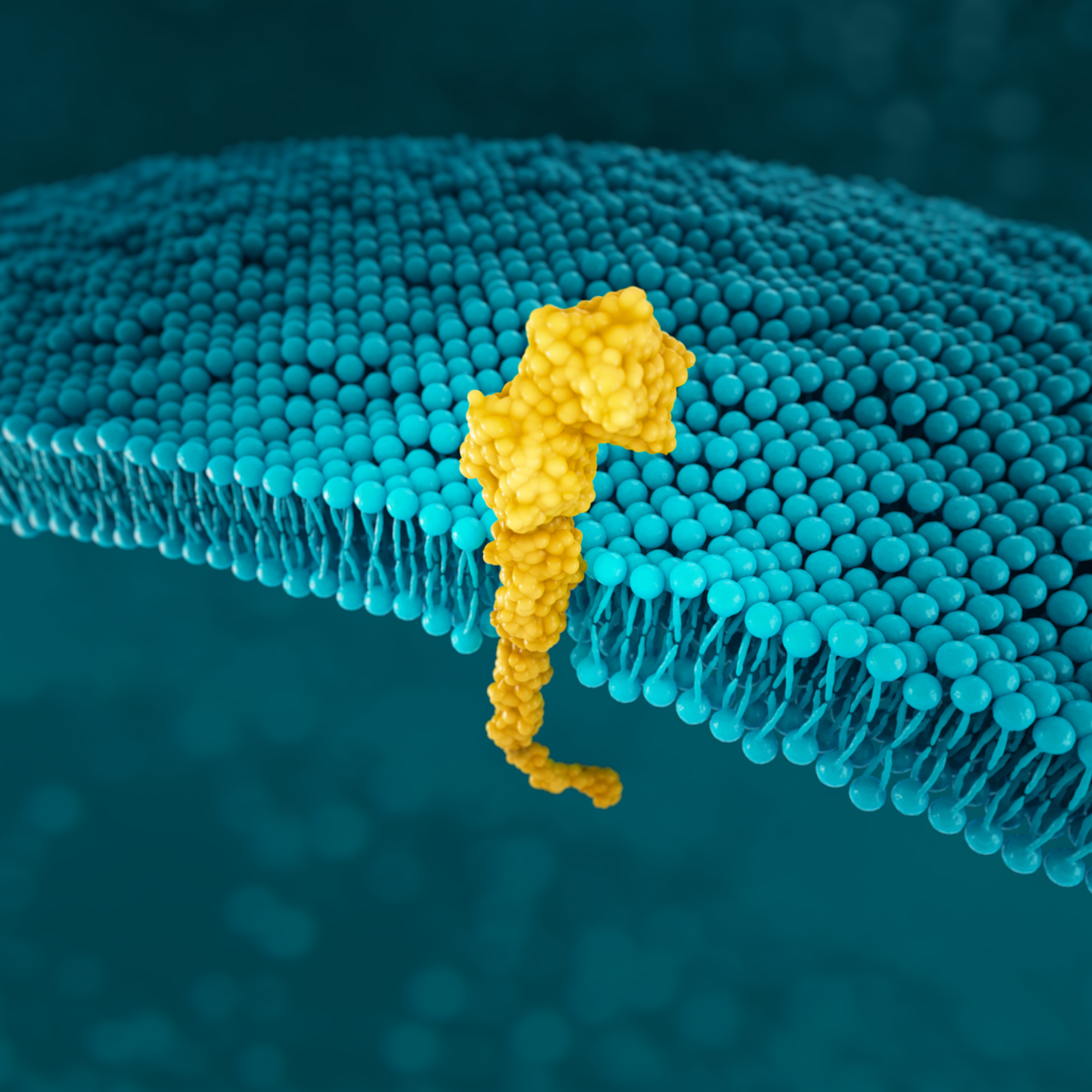
PD-L1
PD-L1 is a transmembrane protein that may be expressed on various tumour cells and/or immune cells.51
As the key regulator of immune tolerance and immune exhaustion,
expression of PD-1 is tightly controlled:53
NORMAL CELL
In normal tissues, PD-1/PD-L1 binding prevents an excessive immune response and protects tissue from damage through the induction of immune tolerance53
GASTRIC CANCER CELL
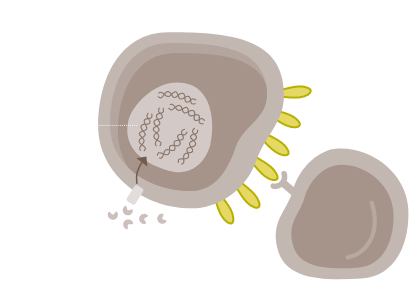
In gastric cancer, CD274 focal amplification and IFN-γ-mediated signalling can lead to PD-L1 overexpression and T-cell exhaustion.53,54
*EBV+ gastric cancer is a distinct gastric cancer subtype defined by EBV infection.
Prevalence of PD-L1 has been reported for several positivity thresholds throughout clinical trials21,23*†






CPS=combined positive score.
*Study population was limited to 592 patients with locally advanced or mG/GOJ cancer who experienced disease progression after first-line therapy with a platinum and fluoropyrimidine.23
†Study analysed 56 specimens from therapy-naïve biopsies from German patients with primarily non-metastatic gastric adenocarcinoma.21
PD-L1 expression is detected using IHC.14
When evaluating the relationship between PD-L1 at two positivity thresholds, data suggest there is limited overlap with CLDN18.2 and HER2 with overlap higher with HER2
| Biomarker | Biomarker | Prevalence overlap | Reference |
|---|---|---|---|
| PD-L1 (CPS ≥1) | CLDN18.2 | 28% | Pellino 202119 |
| PD-L1 (CPS ≥5) | CLDN18.2 | 20% | Pellino 202119 |
| PD-L1+ | HER2 (3+) | 3.2% | Angell 201846 |
| PD-L1+ | MSI | 53.2% | Angell 201846 |
| PD-L1 | FGFR2 | Unknown* | Presently unavailable* |
*A PubMed search using the search terms (gastric cancer) AND (FGFR2) AND (PD-L1) produced five articles, none of which included information pertaining to the prevalence overlap of these two biomarkers.
SUMMARY
Initial diagnostic panels with biomarker testing may lead to more comprehensive patient profiles and informed clinical decisions
Guidelines
The European Society for Medical Oncology (ESMO) guidelines on the diagnosis and treatment of gastric cancer recommend screening for HER2, PD-L1 and MSI-H/dMMR.15,16
NICE recommend HER2 screening for metastatic oesophago-gastric adenocarcinoma.58
CLDN18.2 is a new predictive biomarker for locally advanced unresectable or metastatic gastric adenocarcinoma, but still requiring validation for assessment in a standardised immunohistochemistry (IHC) assay for clinical practice.53,54
FGFR2 amplification/overexpression is being investigated and its validation as a predictive biomarker in randomised controlled trials (RCTs) is awaited.53,54
Biomarker testing provides more insight into mG/GOJ cancer as more biomarkers are being discovered:
*IHC/ISH should be considered first, followed by additional NGS testing as appropriate.¹⁴
As biomarker research continues, it expands our view of the patient population, reveals more information about the mG/GOJ cancer landscape, and helps inform clinical decisions.
Stay up to date
By signing up below to receive more information, you will receive email updates about Astellas Initiatives such as NEW INDICATIONS, PBS LISTING UPDATES, PATIENT SUPPORT MATERIALS, MEDICATION AND DISEASE EDUCATION. Astellas provides medicines and support for doctors treating patients across a range of therapeutic areas. We have healthcare, medical and scientific-related information and materials that we believe may be relevant to your practice.
You will now receive email updates about emerging biomarkers in mG/GEJ cancer, and
you may be contacted by an Astellas representative.
REFERENCES